Case Report
Haema 2019; 10(1):50-55
Vasiliki Palaska,1 Anna Paschali,2 Theodora Triantafyllou,1 Konstantina Keramidioti,1 Evgenia Verrou,1 Nikolaos Drougos,1 Dimitra Markala,3 Polyzo Kazila,4 Persefoni Xirou,5 Asimina Papanikolaou,6 Emmanouil Panagiotidis,2 Elisavet Chadjipavlidou,2 Pavlina Konstantinidou,1 Eirini Katodritou1
1Department of Hematology, Theagenion Cancer Hospital Thessaloniki, 2Department of Nuclear Medicine, Theagenion Cancer Hospital, Thessaloniki; 3Department of Hematology Laboratory Theagenion Cancer Hospital, Thessaloniki, 4Department of Immunology, Theagenion Cancer Hospital, Thessaloniki; 5Department of Pathology, Theagenion Cancer Hospital, Thessaloniki, 6Department of Hemopathology, “Evangelismos” General Hospital, Athens, Greece
Full PDF | ![]()
Abstract
Transferrin receptor 1 (TFRC1) serves as a key regulator of cellular iron homeostasis and proliferation in almost every human cell. Its assignment is vital especially for the normal development and function of the erythroid and neural tissue; Most interestingly TFRC1 holds also a vital role in cancer biology. Soluble transferrin receptor (sTfR) is the truncated form of TFRC1 which reflects the whole erythropoietic mass in healthy subjects and the iron status. In addition, it may be moderately elevated in malignant lymphomas, even in the absence of iron deficiency indicating disease activity. In the current article we present a case of a middle-aged patient with an aggressive diffuse large B cell lymphoma (DLBCL) and extremely high levels of sTfR at diagnosis. During therapy, sTfR levels were reduced following disease response. Of note, sTfR was the only biomarker that displayed gradual increase preceding relapse, which was confirmed by PET CT, suggesting that this biomarker could represent a sensitive tool for early diagnosis of relapse when iron deficiency anemia is excluded.
Key words: sTfR, CD71, TRRC1, DLBCL, PET-CT
Corresponding author: Eirini Katodritou, Hematologist, Director, Hematology Department, Theagenion Cancer Hospital, Alexandrou Symeonidi 2, 54007, Thessaloniki, Greece, e-mail: eirinikatodritou@gmail.com
INTRODUCTION
Transferrin receptor 1 (TFRC1) is a homodimeric protein that serves as a key regulator of cellular iron homeostasis and proliferation;1 its assignment is to form complexes and transfer the molecule of transferrin that is bound to iron molecules (holotransferrin) into the cytoplasm via endocytosis.1,2 After the iron molecules have been removed during the pH degradation in endosomes, the rest of the complex consisting of the molecule of transferrin with the molecule of the transferrin receptor is recycled back to the surface of the cell and a fraction of these complexes are being exocytosised.2 TFRC1 is expressed at a low level in almost every human cell. However, its expression is more profound in rapidly proliferating cells including malignant cells exhibiting thus, a central role in cancer cell biology.1,3 Soluble transferrin receptor (sTfR) is a truncated form of TFRC1 lacking the cytoplasmic and transmembrane domains.4 It exhibits a molecular mass of 85 kDa and forms a complex with transferrin in serum. The main sources of the sTfR are the precursors of erythropoietic cells in the bone marrow.5 The level of sTfR correlates directly with erythropoietic activity and inversely with the amount of iron available for erythropoiesis, providing a quantitative measure of functional iron status.5 Other tissues that contain considerable amounts of TFRC1 such as the liver and the placenta, contribute only slightly to the sTfR pool.5 In healthy subjects sTfR reflects the whole erythropoietic mass, however in most of malignant lymphomas, it was demonstrated that sTfR is usually at some extent elevated even in the presence of abundant iron in bone marrow, probably reflecting the activity of the disease;6 Data regarding the correlation of sTFR with disease stage are limited. It has been demonstrated that patients with early stage lymphomas display lower levels of sTfR compared to those with advanced stage however this was not confirmed between patients in stage III and IV;6 in addition, in the same study, the levels of sTfR in lymphoma patients were statistically higher compared with healthy controls.6 Nevertheless, the implication of sTfR in disease aggressiveness has not been fully investigated. Herein we present a rare case of a middle-aged patient with diffuse large B-Cell lymphoma (DLBCL) presented with unusually high levels of sTfR at diagnosis without any evidence of iron deficiency, which gradually returned to normal in parallel with disease response. The possible role of sTfR as a disease and response biomarker is being discussed.
CASE REPORT
In October 2016, a 49-year old man of a poor performance status was admitted to our department complaining about a rapidly growing swelling in the right cervix, gradual weight loss and night sweats presented within the last 3 months. Physical examination showed a large cervical mass extending to the right axilla and a bulky mass in the left abdomen which could not be discriminated from the spleen. A CT scan revealed the presence of an intracranial mass descending around the Waldeyer’ s ring and deforming bones of the skull and the pituitary gland, a large mass extending from the right cervix to the thorax and a large retroperitoneal mass coming out of the spleen and deforming the left adrenal gland (Figure 1). A cervical lymph node biopsy revealed diffuse infiltration with malignant B-cells; according to immunohistochemistry, malignant cells exhibited positivity for leucocyte common antigen (LCA), CD20/L26, bcl-6 and bcl2, and were negative for c-myc, CD3, CD5, CD10, MUM-1, CD23, CD30, CD138 and cycline D1; moreover, they displayed a high proliferation rate (Ki67: 90%) and interphase fluorescence in situ hybridization (FISH) showed no evidence for c-myc, bcl-2 or bcl-6 translocations. Based on the aforementioned findings a diffuse large B cell lymphoma with centroblastic features, not -otherwise specified, of germinal blastic center origin (DLBCL, NOS, GCB- using the Hans algorithm) was diagnosed. Initial blood tests including complete blood count (CBC) and biochemistry are depicted in Table 1. In addition, anemia and iron status markers, were evaluated as per routine baseline lymphoma protocol (Table 2). Of note, sTfR was extremely high compared to the normal range, in the absence of iron deficiency anemia or hemolysis, therefore we hypothesized that this unusual increase was probably related to the tumor burden. The bone marrow aspiration and trephine biopsy showed infiltration by lymphoma cells and the karyotype was normal. Iron stores in the bone marrow were increased however the incorporation of iron in the erythroblasts was poor. Furthermore, a diagnostic lumbar puncture revealed leptomeningeal infiltration with clonal B-cells. The patient classified as Ann Arbor stage IVXBS with a poor-risk Revised International Prognostic Index (R-IPI: 4).
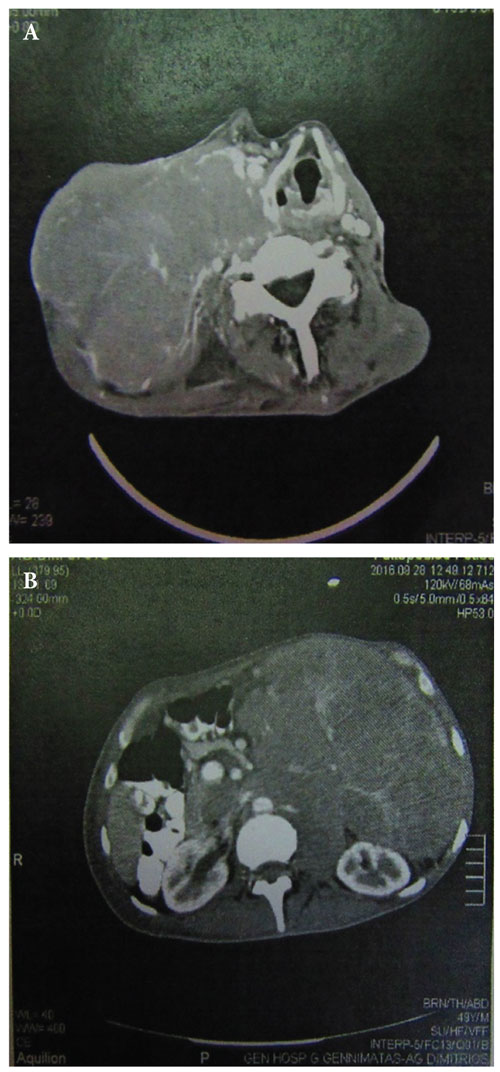
Figure 1. Computed tomography (CT) at diagnosis of the A: Cervix, B: Abdomen.
The patient was treated with rituximab, cyclophosphamide, vincristine, doxorubicin and prednisone (R-CHOP) and therapeutic intrathecal infusions with cytarabine and dexamethazone due to the leptomeningeal infiltration. He also received hormone replacement therapy because of lymphoma-related pituitary gland impairment. After 2 cycles of chemotherapy the clinical condition of the patient improved markedly and according to physical examination there was at least 50% reduction of all the palpable masses in the cervix, axilla and abdomen as well as the spleen. Interestingly, sTfR levels decreased dramatically from 44,4 mg/mL to 4,13 mg/mL and continued to decrease reaching nearly normal after 4 cycles (Figure 3). In addition, a CT scan performed after 4 cycles confirmed a partial response; the bone marrow trephine was normal. The patient received, overall, 8 cycles of R-CHOP and despite the fact that there was still an abnormally enlarged lymph node in the right axilla, a Positron Emission Tomography (PET) scan performed after the end of treatment displayed a borderline metabolic activity (Deauville score 3-4) (Figure 2A), therefore, response was characterized as unconfirmed complete remission (CRu); At the time of CRu sTfR had reached normal levels (Figure 3). Two months later a new PET scan revealed a complete metabolic response without further treatment (CMR) (Deauville score 2) (Figure 2B); at this time point sTfR remained normal (Figure 3). The patient was followed monthly, and three months after the documentation of CMR, sTfR began to increase gradually (Figure 3). Clinical examination did not show any increase of the palpable right axillary lymph node, however, a new PET scan revealed metabolic activity in the right axilla (Figure 2C); in parallel, sTfR continued to increase further (Figure 3). The patient was treated with second line chemotherapy (dexamethasone, ifosfamide, ciplatinum, etoposide; DICE) in order to proceed to peripheral autologous stem cell transplantation. After 2 cycles of DICE a CMR was documented (Figure 2D) and sTfR returned to normal.
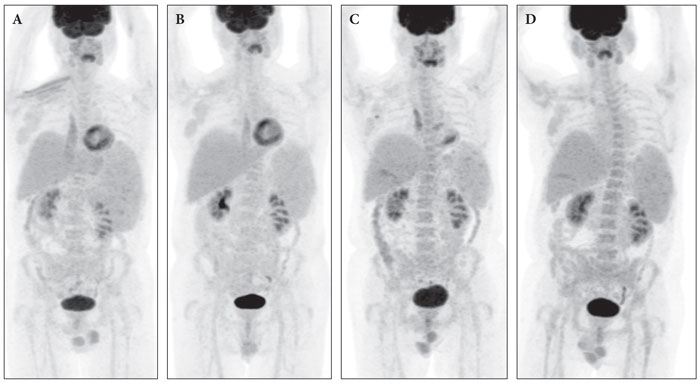
Figure 2. Positron emision tomograpy. A: End of treatment (April-2017. B: follow-up PET June-2017. C: At relapse (September 2017). D: After salvage therapy (December 2017).
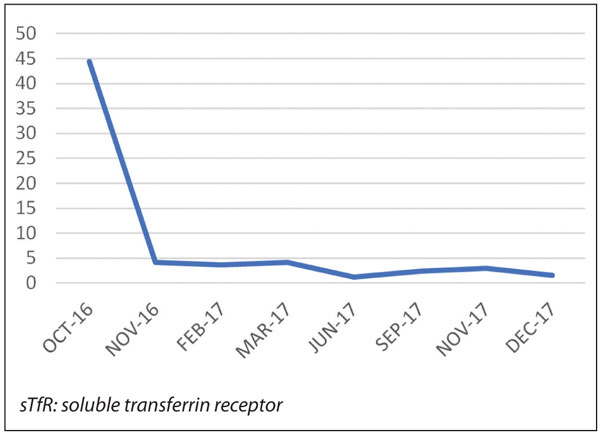
Figure 3. sTfR (mg/L) fluctuations during therapy.
DISCUSSION
Sufficient iron supplement is necessary for cellular functions and proliferation. Therefore, it has been demonstrated that cells with increased iron demands such as cancer cells express higher levels of TFRC1 compared to other normal cells in order to cover iron requirements.1 Herein we present a case of a patient with a poor-prognosis diffuse large B-cell lymphoma presented with bulky disease with concomitant unusually high increase of the sTfR levels, without evidence of iron deficiency anemia or hemolysis. In previously published studies it was demonstrated that in malignant lymphomas, sTfR level may be elevated and reflect the activity and stage of the disease rather than iron status in malignant lymphomas.6-8 Furthermore, Hindawy et al showed that sTfR was higher in newly diagnosed or relapsed patients with malignant lymphomas compared to patients in remission.6 In the study performed by Stasi et al including newly diagnosed patients with aggressive NHLs it was demonstrated that sTfR could serve as a marker of monitoring response to treatment and early relapse.9 In the study conducted by Bjerner et al,7 sTfR was elevated in a considerable proportion of patients with malignant lymphomas, but the magnitude of these elevations was small: It was less than 2x in all cases with abnormal values except one, in which sTfR concentration was approximately 7 mg/L, i.e. <3x. The novelty of this case report lies on the observation of exteremely high baseline levels of sTfR (>15-20x) compared to all previously mentioned studies, reflecting the aggressiveness of the disease confirmed by the extremely high tumor burden and disease extent as well as a very high proliferation rate based on Ki67 expression. Interestingly, the metabolic activity of the tumor expressed by the intake of 2-deoxy-2-[fluorine-18]fluoro- D-glucose by the malignant cells demonstrated with the PET-CT was also reduced in parallel with the reduction of sTfR, supporting the possible role of this marker in monitoring disease response. In addition, in the current case, a gradual increase of sTfR preceded lymphoma relapse, which was documented soon after by PET-CT. At variance, LDH and β2-microglobulin did not show any increase indicative of relapse at that time point (data not shown); this suggests that sTfR evaluation is a simple blood test which could guide physicians to suspect relapse in the absence of iron deficiency, even in case there is no other clinical or biochemical evidence. In such case, sensitive imaging techniques such as PET/CT could be performed in order to confirm or exclude relapse.
Recent scientific work demonstrated in vivo and in vitro models of B cell lymphoma that expression of the TFRC1 is upregulated by the c-Myc which interferes also with other factors of iron metabolism making TFRC1 an important mediator of the c-Myc proliferating capacity, but not a factor correlating with the capacity for cell growth. Conversely inhibition of TFRC1 and subsequently depletion of iron leads to G1 arrest but does not affect cell size. Expression Analysis Systematic Explorer (EASE) revealed that TFRC1 inhibition promoted altered expression of genes correlating with the cell cycle and the programmed death and that these genes were also upregulated in cells with low c-myc expression.3 On the other hand, except for the cancer cells, c-Myc is also produced in small amounts at some time of the cell cycle of every normal not terminally differentiated cell and it is essential for casting its proliferating capacity before being totally catabolized. In the current case, despite the extremely high levels of sTfR, formalin fixed paraffin embedded specimen was not stained positive for c-Myc indicating that even undetectable levels of c-myc are able to promote TFRC expression. In the present case, we were not able to confirm TFRC1 expression on malignant cells, due to technical problems during staining procedure, however the gradual reduction of sTfR in parallel with the regression of the tumor burden, proved by sequential PET-CT imaging confirms the relation of TFRC with the malignant clone. Moreover, even small changes in metabolic activity of the tumor produced detectable change in sTfR, representing thus a possible early marker of relapse.
The gene for TFRC1 is located in 3q29. Moreover, other proteins that are involved in iron metabolism like transferrin and ceruloplasmin are also located in the chromosome 3. Gain of the 3q arm or 3q27qter or trisomy of chromosome 3 have been found in some hematopoietic or solid tumor malignancies.10 Considering the above data, we performed a conventional karyotype in order to seek for possible abnormalities especially in chromosome 3, however, karyotype was normal. Nevertheless, the role of these karyotypic abnormalities and their correlation with iron uptake remains unclear although research shows that ectopic expression of TfRC1 constitutes a vital advantage in the tumor microenvironment.3
In summary, sTfR may reflect disease activity especially in aggressive malignant lymphomas. Occasional patients may have extreme sTfR elevations and sTfR could be used as a marker to evaluate response during treatment. Most interestingly, sTfR alterations were recorded in parallel with the conventional imaging and PET-CT findings, indicating that sTfR could also serve as an early surrogate marker of disease relapse guiding the decision for performing additional tests such as modern imaging, in order to detect disease recurrence as soon as possible.
Conflict of Interest
None.
REFERENCES
1. Ponka P, Lok CN. The transferrin receptor: Role in health and disease. Int J Biochem Cell Biol. 1999 Oct;31(10):1111-37.
2. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329-39.
3. O’Donnel KA, Yu D, Zeller KI, Kim JW, Racke F, Thomas-Tikhonenko A, et al. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol Cell Biol. 2006 Mar;26(6):2373-86.
4. Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997 Feb;89(3):1052-7.
5. Suominen P, Punnonen K, Rajamäki A, Irjala K. Evaluation of new immunoenzymometric assay for measuring soluble transferrin receptor to detect iron deficiency in anemic patients. Clin Chem. 1997 Sep;43(9):1641-6.
6. Hindawy D, Akl S, El-Mahallawy H, Abd El-Latif NAB, El-Attar I. Serum transferrin receptor in relation to iron status in different disease stages of adult malignant lymphoma. Journal of the Egyptian Nat. Cancer Inst. 2002 Sep;14(3):237-42.
7. Bjerner J, Amlie LM, Rusten LS, Jakobsen E. Serum levels of soluble transferrin receptor correlate with severity of disease but not with iron stores in patients with malignant lymphomas. Tumour Biol. 2002 May-Jun;23(3):146-53.
8. Stasi R, Zinzani L, Galieni P, Lauta VM, Damasio E, Dispensa E, et al. Clinical implications of cytokine and soluble receptor measurements in patients with newly-diagnosed aggressive non-Hodgkin’s lymphoma. Eur J Haematol. 1995 Jan;54(1):9-17.
9. Das Gupta A, Shah VI. Correlation of transferrin receptor expression with histologic grade and immunophenotype in chronic lymphocytic leukemia and non-Hodgkin’s lymphoma. Hematol Pathol. 1990;4(1):37-41.
10. Tevfik Dorak M. TFRC (transferrin receptor (p90, CD71). Atlas Genet Cytogenet Oncol Haematol. 2009; 13: 222-224 Free journal version. Available from: http://AtlasGeneticsOncology.Org/Genes/TFRCID259ch3q29.html
Received 15 Jan 2019
Accepted 4 Feb 2019