Haema 2019; 10(2):116-127
Konstantinos Liapis
Consultant Haematologist, Georgios Gennimatas Hospital
Full PDF | ![]()
Asymptomatic individuals living in the tropics or migrants originating from tropical countries often have increased eosinophil counts due to subclinical infections by helminths; counts are higher in rural and non-elite groups than in urban and elite groups. In the tropics, eosinophilia is more likely to be a response to helminthiasis than to be an indication of allergy.
In any traveller returning from tropical and subtropical areas with eosinophilia a helminth infection should be ruled out. On questioning, it is of course important to make a temporal relationship of eosinophilia with travelling, but keep in mind that eosinophilia can occur with a significant delay.
History1-6
The travel history should include recent (within 3 months) and past travel, since some important causes of eosinophilia such as Strongyloidiasis or schistosomiasis may present after years, even decades from exposure.
It is also crucial to get a detailed history of possible exposures and concomitant symptoms (a so-called aetiologic inquiry). Exposure to food items (traditional food, salads, watercress, crabs, raw fish, undercooked meat, snails, frogs) should be explicitly enquired after. The occurrence of similar symptoms or eosinophilia in a person who participated in the same meal is a strong indication of a food-borne nematode.
Patients should be asked about hygiene, freshwater exposure e.g. swimming or fishing in lakes or rivers (schistosomiasis) and contact with sand or soil (soil-transmitted helminths, Fasciola hepatica, and other flukes) and pets. However, negative exposure history does not rule out risk, as many patients simply do not recall their exposure.
Don’t forget to ask about previous blood counts, drugs (make a list of all drugs and over-the-counter medications for the last 3 months), profession, personal or family history of atopy (asthma, allergic rhinitis, eczema), and family history of eosinophilia.
Pyrexia and concomitant symptoms may also help to narrow the differential diagnosis. However, eosinophilia is asymptomatic in up to one-third of returning travellers and migrants. Common causes of asymptomatic eosinophilia include: intestinal helminths (following migration of larvae through tissues), schistosomiasis, and filariasis.
Clinical aspects of eosinophilia
Eosinophilia is classified as: mild (0.5-1.5×10⁹/l), moderate (1.5-5.0×10⁹/l), and severe (>5.0×10⁹/l). Hypereosinophilia (HE) is defined as an eosinophil count >1.5×10⁹/l on two examinations with an interval of >1 month. Hypereosinophilic syndrome (HES) is defined as hypereosinophilia and organ damage mediated by eosinophils. Cases with HE of unknown cause and no organ damage are called idiopathic hypereosinophilia (iHE) or HE of unknown significance (HEUS).7
Allergies, atopic dermatitis, asthma: in general, eosinophils are <1.5×109/l although they may reach hypereosinophilic levels is some patients, especially children and young adults (however, if eosinophils are ≥5.0×109/l you should look for a different cause even in atopic people).
Helminthic infections: often moderate (1.5-5.0×109/l) to severe (>5.0×109/l) eosinophilia. The eosinophil count can be very high (>20×109/l). Therefore, we cannot rely upon the presence of severe eosinophilia, even counts >20×109/l, to distinguish hypereosinophilic syndrome (HES) from helminthiasis!
Note: 1. allergic symptoms (e.g. urticarial rash, rashes of various kinds, allergic dermatitis, eczema, pruritus, bronchospasm) are common in patients with helminthic infection 2. allergic disorders are common manifestations of idiopathic HES and L-HES 3. any allergic individual may present with an additional cause, reactive or neoplastic, for eosinophilia.
Drugs can cause significant eosinophilia, even severe, which may or may not be accompanied by hypersensitivity symptoms, particularly rash, a systemic reaction with fever (DRESS), increased liver enzymes (hypersensitivity hepatitis), or lung infiltrates (hypersensitivity pneumonitis). DRESS is characterised by a triad of skin rash (typically appears ≥3 weeks after starting a medication and is often associated with facial oedema), fever, and organ involvement (liver, lymph nodes, lung, kidneys, or heart). Diagnosis depends upon history (drug exposure and its temporal relationship with eosinophilia).
Some cases of DRESS occur in association with herpesvirus infection (HHV-6, EBV, or CMV), either primary or reactivated (suspect HHV-6 in DRESS with lymphadenopathy and atypical lymphocytosis). It is possible that HHV-6 is the trigger for DRESS syndrome in the context of a drug exposure. Atypical lymphocytes resembling virally activated lymphocytes and eosinophilia may also be seen in drug hypersensitivity without HHV-6, serum sickness, and L-HES.
Drugs associated with eosinophilia include antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), antiepileptics, and others (allopurinol, salazopyrin, abacavir). Eosinophilia caused by drugs is usually benign but can sometimes be accompanied by tissue damage, as in hypersensitivity pneumonitis. Sometimes atypical lymphocytes are present in the peripheral blood. In most cases, eosinophilia resolves once the drug is withdrawn.8-12
Terminology of helminths:
Helminths (worms) = nematode helminths (roundworms) and platyhelminths (flatworms) which include cestodes (tapeworms) and trematodes (flukes).
In helminths, the eggs (ova) may become infectious only after maturation in moist soil (e.g. Ascaris), or after passing through an intermediate host (e.g. Trichinella).
Helminthiases acquired from the soil (soil-mediated) include Ascaris, hookworm infections, Toxocara, and Strongyloides; those requiring a snail intermediate host (snail-mediated) include schistosomiasis, the liver flukes, and Angiostrongylus (rat lungworm).
Helminths presenting in travellers and migrants with eosinophilia:1-6,13-17
- Nematodes
Ascaris lumbricoides: more common and higher eosinophil counts in children and young adults. The migration of larvae from the small intestine to the lungs and bronchi during acute infection may cause Loeffler’s syndrome, a form of eosinophilic infiltration of the lungs manifesting as cough, low grade fever, malaise, peripheral eosinophilia, and transitory pulmonary infiltrates usually lasting for 7-10 days (transitory is defined as lasting less than 15 days). Loeffler’s syndrome can also occur from the larval migration through the lungs of the larvae of other nematodes such as Strongyloides. Diagnosis depends on stool microscopy and/or serology.
Toxocara canis, Toxocara cati: transmitted by ingestion of eggs contained in infected faeces of dogs or cats (especially puppies and kittens, which are more likely to carry the infection). Infection with Toxocara can cause visceral larva migrans a syndrome with fever, GI symptoms, hypereosinophilia, and local tissue damage e.g. liver involvement with hepatomegaly and hypodense lesions on CT (ddx: abscesses) or lung infiltration. Visceral larva migrans occurs when larvae from ingested toxocara eggs penetrate the gut mucosa and enter the portal and then the systemic circulation. The most consistent finding in visceral larva migrans has been a leukocytosis with marked eosinophilia. Leukocytes usually range from 12-100×109/l with eosinophils making up 15-80%. Note: toxocara can cause extreme eosinophilia >85×109/l and marked hypergammaglobulinaemia (IgG, IgM, IgE). Occular larva migrans is a distinct syndrome without eosinophilia. Stool exam is negative and diagnosis depends on serology.
Filariasis: frequently is associated with significant eosinophilia, especially L. loa. In loiasis, the eosinophil count is often 3-7×10⁹/l but may be higher. W. bancrofti and B. malayi are associated with tropical pulmonary eosinophilia, a hypersensitivity-allergic reaction with bronchospasm, nocturnal cough, dyspnea, interstitial infiltrates, and marked peripheral eosinophilia (>3.0 ×10⁹/l) more often seen in visitors to than long-term inhabitants of endemic regions, and lymphatic filariasis in which there is not always eosinophilia. Eosinophilia may be the only feature of filariasis. Diagnosis is made by blood film examination and/or serology.
Ancylostoma duodenale, Necator americanus (hookworm infections): the most common cause of eosinophilia in returning travellers or migrants from tropical and subtropical countries (worldwide distribution). The eosinophil count is often ~2.0×109/l. Diagnosis depends on stool microscopy and/or serology.
Strongyloides stercoralis: an important cause of eosinophilia. It is the most common cause of eosinophilia in returning servicemen. Subclinical infection with S. stercoralis can persist for >20 years. Usually asymptomatic. A raised eosinophil count may be the only feature (but immunosuppression can make the eosinophil count falsely normal). Stool exam is often negative and diagnosis usually depends on serology.
Τrichinella spiralis: caused by the ingestion of larvae encysted in muscle (through undercooked pork or pork products eaten several days before onset of symptoms). An ‘enteral phase’ with GI symptoms, as the ingested larvae mature to adults and produce larvae in the intestinal tract, is followed by a ‘parenteral phase’ as the larvae migrate from intestine through circulation to muscle, where they encyst. Eosinophilia (often >3.0×10⁹/l) and fever occur mostly during larval migration. The four cardinal features of the disease are fever, orbital oedema, myalgia, and eosinophilia. An urticarial rash may be present. Complications include heart failure and rarely CNS involvement. Diagnosis depends on serology.
Other helminthiases: Trichuris trichiura, Capillaria philippinensis, Gnathostoma, Paragonimus spp., Anisakis spp.
Note: Enterobius vermicularis, the most common human helminthiasis, does not cause eosinophilia because it is not tissue-invasive (except for rare cases with an enormous burden of worms).
- Cestodes (tapeworms)
Echinococcus: eosinophilia is present in 25% of patients with hydatid disease. Echinococcosis is particularly common in the Middle East (Anatolia Turkey), North and East Africa, central Asia, and South America. The presence of eosinophilia may indicate cyst leakage. Diagnosis depends on serology (but not always positive) and compatible US or CT appearance. Serological cross-reactions giving rise to false-positive results can occur with other parasitic infections notably larval cestodes and filarial worms and with some neoplasms. False negatives may occur, and are more common in the case of non-hepatic hydatid cysts.
- Trematodes (flukes)
Fasciola hepatica: adult flukes live in the bile ducts where they cause serious chronic damage to the biliary tree (biliary obstruction, cholecystitis, cholangiitis, and liver abscess). Eosinophilia is mainly seen in acute fascioliasis. The classic picture of acute fascioliasis is fever, right upper quadrant pain, abnormal LFTs ± jaundice, hypodense lesions on CT (ddx: abscesses), and marked eosinophilia (which may be extreme). Commonest in individuals returning from the Middle East (including Turkey). It is also endemic in Egypt and the Andes (Βolivia, Peru). Stool microscopy usually has low sensitivity; diagnosis usually depends on serology.
Clonorchis sinensis (also known as the Chinese or Oriental liver fluke) and Οpisthorchis viverrini: similar clinical picture to F. hepatica in people from South-East Asia, China, and Japan. In people from northern and eastern European countries e.g. Ukraine, Οpisthorchis felineus may cause a very similar illness with eosinophilia (Ο. felineus has also been found in northern-western Greece). Diagnosis is made by stool microscopy.
S. mansoni, S. haematobium, S. japonicum
Schistosomiasis (river disease)1-6,13,14
One of the most common and important causes of moderate to high eosinophilia globally. Infection with this parasite can be:
acute schistosomiasis: diarrhoea and/or acute schistosomal colitis in heavy infections and/or Katayama syndrome with fever, eosinophilia (which may be high grade >5×109/l), dry cough, interstitial or nodular pulmonary infiltrates, and sometimes an uricarial rash. Katayama syndrome is seen mainly in Africa and occasionally in South-East Asia.
chronic schistosomiasis due to S. mansoni (high incidence in the Great Lakes of East and southern Africa e.g. in Malawi and Mozambique and also in Cameroon, Egypt, and Brazil), or due to S. japonicum (high incidence in South-East Asia, the Philippines, China, and Japan) causes periportal fibrosis (‘pipestem fibrosis’) → portal hypertension → oesophageal varices and massive splenomegaly (the combination of enlarged, irregularly fibrosed liver and greatly enlarged spleen has been called ‘Egyptian hepatosplenomegaly’ or hepatosplenic schistosomiasis).
Note: an eosinophilic response to S. mansoni may not be apparent in the peripheral blood count because the eosinophils are held in the spleen, but it will be obvious in the bone marrow aspiration done for investigation of splenomegaly.
chronic schistosomiasis due to S. haematobium (African Great Lakes, Egypt) is associated with urogenital involvement (haematuria, often at the end of urination is a characteristic symptom). Haematuria can lead to iron deficiency anaemia.
Note: an outbreak of locally acquired urogenital schistosomiasis occurred in tourists in Corsica in 2014.
The geographic distribution of S. mansoni is shown in Figure 1 and of S. haematobium and S. japonicum in Figure 2.
The diagnosis depends upon maintaining a high index of suspicion for schistosomiasis in all travellers to tropical and subtropical areas with eosinophilia (obtain a travel history and a history of freshwater exposure).
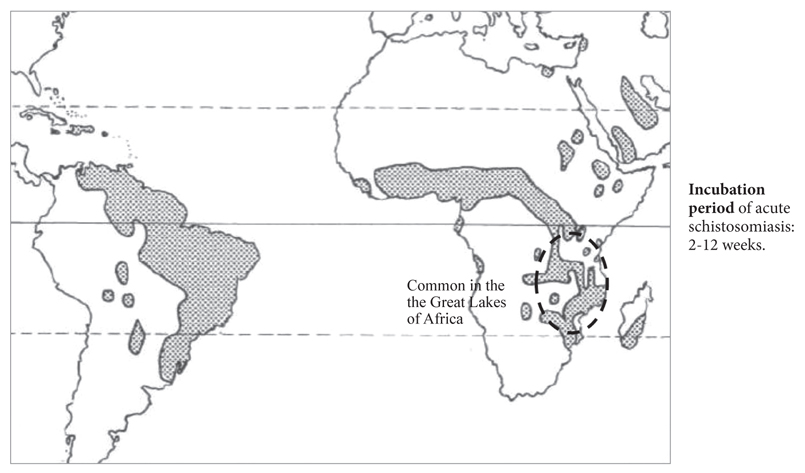
Figure 1. Distribution map of Schistosoma mansoni.
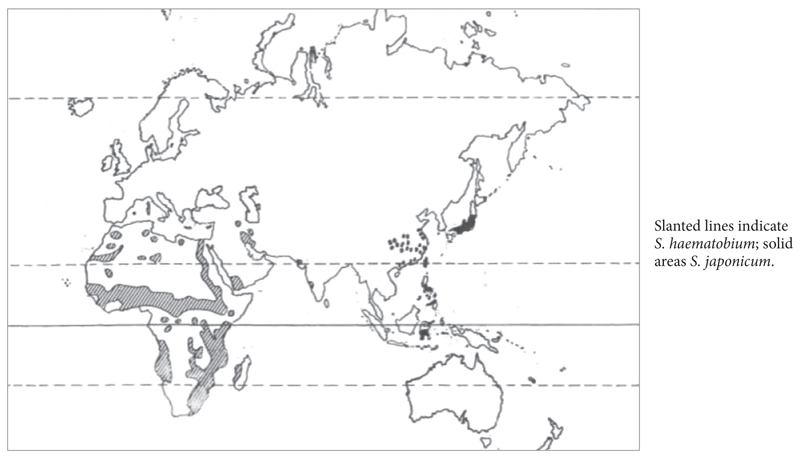
Figure 2. Distibution map of S. haematobium and S. japonicum.
The diagnosis of schistosomiasis is made by:
– stool microscopy and urine microscopy for ova ×3; it is often negative (low sensitivity)
– antibodies: serology is more sensitive than microscopy, but may be false negative e.g. in Katayama syndrome because Abs may take up to 3 months to turn positive à a negative result is not enough and should be repeated!
– an effective test is a rectal biopy through a sigmoidoscope or liver biopsy to find schistosome ova (and/or bladder biopsy for S. haematobium). Rectal or bladder biopsy for the identification of eggs is usually performed if stool or urine are egg-negative but schistosomiasis is still suspected. Note: eggs of Schistosoma may lodge ectopically in any tissue, where they cause characteristic granulomas.
Strongyloidiasis1-6,16,17
S. stercoralis is found in tropical Africa, South America, South-East Asia (where prevalence may exceed 20%), in Egypt, parts of Asia (e.g. Pakistan, India), and the Caribbean (Figure 3). S. stercoralis infection may cause diarrhoea and sometimes a characteristic cutaneous creeping eruption especially in the back (larva currens), due to migrating larvae of S. stercoralis in the skin (ddx: cutaneous larva migrans). This may occur many years (30 or more) after initial infection. Deep migration of the larvae may be associated with an ‘eosinophilic lung’ type of syndrome (Loeffler’s syndrome). Most cases of strongyloidiasis, however, are asymptomatic or cause only mild symptoms. Eosinophilia, the most important finding, may be mild, moderate, or severe, but may also be absent.
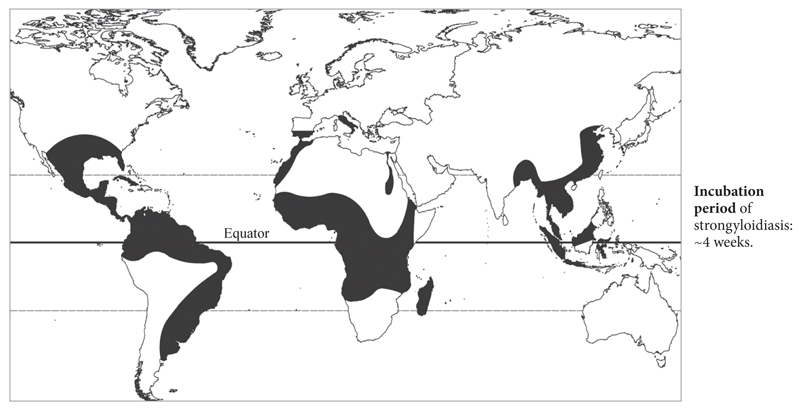
Figure 3. Distribution map of Strongyloides stercoralis.
S. stercoralis is very important for immunocompromised individuals e.g. corticosteroids, chemotherapy*, haematological malignancies, transplantation, and infection with HTLV-1. The association of strongyloides with HTLV-1 is especially common in West Africa and the Caribbean. In immunocompromised hosts, strongyloidiasis may disseminate and cause overwhelming life-threatening disease (hyperinfection syndrome) due to massive migration of larvae and/or secondary Gram negative septicaemia. Hyperinfection with S. stercoralis is the accumulation of a large burden of parasite.
In HIV infection, Strongyloides hyperinfection syndrome has been described but is less common than HTLV-1. Why? — In HTLV-1, the Th1 program is enhanced, and the Th2 response is blunted, resulting in impaired ability to defend against helminths. In HIV, loss of CD4+ T-cells can affect both the Th1 and the Th2 responses, and in some patients a Th2 response may even predominate. Therefore, the Th2 response is not as disproportionately blunted in patients with HIV as it is in patients with HTLV-1 infection.
* e.g. in a patient with newly-diagnosed multiple myeloma who lives in Athens for 15 years but was born in Pakistan with mild eosinophilia (0.8×109/l) and a history of allergic rashes à strongyloidiasis must be ruled out before initiation of chemotherapy.
The diagnosis depends upon maintaining a high index of suspicion for stongyloidiasis.
Diagnosis is made by detection of living rhabditiform larvae in stool specimens (it is very unusual to find ova); however, stool microscopy is often false-negative due to intermittent larval excretion and/or low burden. Serology is usually positive, but Abs become positive only 4-12 weeks post-infection so that Abs may be negative when eosinophilia and/or larva currens are first detected. False-positive serology may occur due to cross-reacting antibodies against other helminths, particularly nematodes. Strongyloides serology may be negative in the Strongyloides hyperinfection syndrome.
Travellers and migrants from West or Central Africa e.g Cameroon with eosinophilia and positive Strongyloides tests, should be screened for Loa loa co-infection (daytime blood film) prior to receiving ivermectin. In patients unable to receive ivermectin (significant microfilaraemia+), albendazole 400 mg twice daily for three days is an alternative.
Note: albendazole is safe and active against all nematodes and against cestodes (Echinococcus) as well as moderately active against Fasciola. The best drug for most trematode infections is praziquantel.
Serological tests (ELISA) are available for all helminths mentioned above. The serological tests are very useful for Strongyloides, Toxocara, Trichinella, Schistosoma, and filariases. Note: false-positive serological tests may occur due to cross-reacting antibodies between nematodes e.g. between Strongyloides and Ascaris. The serological tests for L. loa use antigens obtained from Dirofilaria immitis, a filaria species that infects dogs, and therefore cannot distinguish between different human filarial species (non-specific). Filarial antigen and PCR for filarial DNA are also available.
How is it diagnosed?
filariasis? → day and night blood films +/- serology
strongyloidiasis? → stool microscopy + serology
rest nematodes? → stool examination +/- serology
schistosomiasis? → stool and urine microscopy + serology +/- rectal biopsy
rest trematodes? → stool microscopy + serology
Note: serum IgE is typically very high in eosinophilia due to helminthiasis. A raised IgE is sensitive but non-specific. IgE is also high in allergic/severely atopic individuals, drug-induced eosinophilia, the lymphocytic variant of HES (L-HES), some patients with the myeloid variant of HES (Μ-HES) or paraneoplastic eosinophilia (e.g. Hodgkin’s lymphoma and T-cell lymphoma), cases of isolated eosinophilic organ infiltration associated with peripheral eosinophilia (so-called eosinophil-associated single-organ disease e.g. eosinophilic gastroenteritis), Churg-Strauss syndrome, allergic bronchopulmonary aspergillosis, IgE multiple myeloma, and the hyper-IgE syndrome or Job’s syndrome (a hereditary immunodeficiency disorder with impaired neutrophil chemotaxis, markedly raised IgE typically >2000 IU/ml, and often eosinophilia). High IgE can occur in schistosomiasis even without eosinophilia. Paraneoplastic eosinophilia can be associated with markedly raised IgE e.g. 2930 IU/ml in a patient with peripheral T-cell lymphoma. Normally, serum IgE is <180 IU/ml.
Tip:
– give albendazole (albendol) 400 mg bd for 3 days in patients with:
– eosinophilia
– larva currens
– (+) serology for strongyloides
– possible exposure to S. stercoralis (e.g. close contact with a person with confirmed strongyloidiasis)
Malaria does not cause eosinophilia, but may co-exist with helminthiasis in tropical regions (e.g. P. falciparum with Loa loa or strongyloides in tropical Africa), so malaria must be ruled out with a blood film in any patient who has stayed in the tropics and presents with fever and eosinophilia.
Eosinophilia associated with specific symptoms in the tropics:1-5,13
– eosinophilia + fundal changes (chorioretinitis) → Τoxocara, Toxoplasma (eosinophilia is uncommon in toxoplasmic chorioretinitis), vasculitis.
– eosinophilia + myalgia or high CPK → Τrichinella, eosinophilic myositis, ΗΕS, eosinophilia-myalgia syndrome (severe myalgia and eosinophilia but CPK typically normal or minimal elevation), collagen vascular diseases.
– eosinophilia + iron deficiency anaemia → Ancylostoma duodenale, Τrichuris, schistosoma, eosinophilic gastroenteritis, GI tract malignancy (lymphoma, Ca).
– eosinophilia + splenomegaly → schistosomiasis, filariasis, toxocara, lymphoma, leukaemia, CML, MPN, CEL, HES, mastocytosis.
– eosinophilia + abnormal liver tests + fever → Katayama syndrome, Fasciola, Clonorchis, Οpisthorchis, Toxocara, Echinococcus, lymphoma, mastocytosis, Ca, hepatoma, cholangioCa.
– eosinophilia + lymphadenopathy→ lymphatic filariasis, loiasis, lymphoma, leukaemia (rule out co-existent strongyloides), Κimura disease (in Asia: India, China, Korea, Japan), L-HES, mastocytosis, DRESS, Ca, histiocytosis (mainly in children).
– eosinophilia + urogenital symptoms → schistosomiasis, lymphatic filariasis, eosinophilic cystitis.
– eosinophilia + testicular tenderness or hydrocele → lymphatic filariasis, S. haematobium, lymphoma, polyarteritis nodosa.
– eosinophilia + diarrhoea or abdominal pain + fever → Strongyloides, Τoxocara, Τrichinella, Αnisakis, Schistosoma, Αscaris, Trichuris, eosinophilic gastroenteritis, ΗΕS, Churg-Strauss, polyarteritis nodosa, inflammatory bowel disease, mastocytosis, GI lymphoma or Ca.
– eosinophilia + eosinophilic meningitis (definition of eosinophilorrachia: eosinophils >10% or >10/μl in CSF) → Angiostrongylus cantonensis (most important cause), Gnathostoma spp., Loa loa, Toxocara, neurocysticercosis, lymphoma, leukaemia (meningeal involvement), ΗΕS.
– eosinophilia + spinal cord compression (paraplegia) → schistosomiasis, lymphoma, Ca.
– eosinophilia + migrating or creeping cutaneous lesion → cutaneous larva migrans (caused by Ancylostoma caninum: dogs and A. braziliense: cats; common in South and Central America, the Caribbean, South Asia, and South-East Asia; eosinophilia is rare), larva currens (Strongyloides), Gnathostoma spinigerum (South-East Asia and East Africa), Loa loa (mainly subcutaneous swellings), sparganosis (in East Asia, Kenya, and Tanzania; rare).
– eosinophilia + transient subcutaneous swellings on upper or lower extremities → Loa loa (Calabar swellings), Mansonella perstans. Dirofilaria repens, a dog filaria, that may infect humans through mosquitoes in Europe, especially South Europe (Italy, Spain, Greece) can cause migratory subcutaneous nodule or transient subcutaneous swellings e.g. in the face or elsewhere without eosinophilia (because there is no microfilaraemia). Lymphoma and polyarteritis can cause eosinophilia and subcutaneous nodules or swellings which are not transient.
Tip: in patients with the triad of transient swelling of the hand or wrists, eosinophilia, and a stay in Africa (e.g. Cameroon) → search the blood film for Loa loa.
eosinophilia + eosinophilic ascites (definition: >10% eosinophils in ascitic fluid) → eosinophilic gastroenteritis (most important cause), Strongyloides, Toxocara, Fasciola, HES, intra-abdominal lymphoma, Ca.
eosinophilia + helminth in the eye →Loa loa. The dog parasites Dirofilaria repens (in Europe) and Thelazia callipaeda (in Asia and Europe e.g. Italy, France, Spain, and the Balkans which is transmitted through drosophila fruit fly) may cause a similar picture in the eye but without eosinophilia (no microfilaraemia).
eosinophilia + respiratory symptoms or lung infiltrate(s) ± fever (definition of pulmonary eosinophilia: >10% eosinophils in bronchoalveolar lavage) → Katayama fever, Loeffler’s syndrome, tropical pulmonary eosinophilia, Τοxocara, Paragonimus spp., Echinococcus, endemic mycoses, Churg-Strauss syndrome, acute and chronic eosinophilic pneumonia, ΗΕS, drug hypersensitivity, lymphoma, allergic bronchopulmonary aspergillosis, eosinophilia-myalgia syndrome, sarcoid, histiocytosis.
eosinophilia + eosinophilic pleuritis (definition: >10% eosinophils in pleural fluid) → pulmonary eosinophilia (Loeffler’s syndrome, lymphatic filariasis), Paragonimus spp., Echinococcus, collagen vascular disease, lymphoma, histiocytosis, Ca.
Non-infective causes should also be considered in returning travellers and migrants, particularly if the eosinophilia is persistent. Helminthiasis and haematological disease are the most important considerations in the differential diagnosis of eosinophilia in the tropics. The haematological causes are as in non-tropical regions with the exception of adult T-cell leukaemia/lymphoma (ATL) which is found in the West and Central Africa and the Caribbean.
Haematological causes of eosinophilia >1.5×109/l in the tropics* 9-12,18-20
– Leukaemia and lymphoma, especially ATL, Hodgkin’s lymphoma (eosinophilia is seen in 15% of patients with Hodgkin’s lymphoma), Sézary syndrome/mycosis fungoides (38% of patients with Sézary syndrome or advanced mycosis fungoides), AITL (20%), other T-cell lymphomas, ALL (‘eosinophilic’ ALL)
– Chronic eosinophilic leukaemia (CEL): blast cells in peripheral blood ≥2% and/or bone marrow >5% and/or cytogenetic or molecular abnormality**
– MPN, MDS/MPNs (aCML,CMML)**
– CML**
– HES: ~25% M-HES**, ~25% L-HES, ~50% idiopathic HES (Table 1)
– Systemic mastocytosis is rarely associated with hypereosinophilia (Table 1)**
– Histiocytosis (rare cause of hypereosinophilia)
* an underlying concomitant helminthiasis should be always ruled out, especially strongyloidiasis and filariasis. A practical solution is to give albendazole before chemotherapy.
** may transform to AML
M-HES
– M-HES is a multi-system disease unlike the syndromes of isolated eosinophilic organ involvement with peripheral eosinophilia (eosinophil-associated single-organ disease) e.g. eosinophilic gastroenteritis which never progresses to systemic disease with cardiac involvement.
– High fevers (>38oC) or prominent GI symptoms are uncommon in M-HES ≠ helminthiases (GI involvement with diarrhoea and abdominal pain occurs in 20% of M-HES).
– The eosinophil count may be extreme (>100×109/l), but extreme eosinophilia and leukaemoid reactions may also be seen in helminthiases (e.g. Toxocara and acute fascioliasis), Churg-Strauss syndrome, and drug hypersensitivity reactions.
– Unlike helminthiases, serum IgE is usually normal (however, occcassional patients with M-HES have raised IgE).
– The morphology of peripheral blood is very useful in M-HES because it may show many abnormal eosinophils (dysplastic eosinophils), including:
– eosinophils with a trilobed nucleus or hypersegmented eosinophils (the most common finding; hypersegmented eosinophils may also be a feature of vitamin B12 or folic acid deficiency)
– unilobed eosinophils (nuclear hyposegmentation)
– eosinophils with reduced granulation (hypogranulation) or sparse granulation with clear patches of the cytoplasm without granules (abnormal granulation)
– eosinophils with smaller than normal granules (microgranulation)
– eosinophils with many cytoplasmic vacuoles (vacuolisation) due to degranulation (common finding in M-HES but non-specific)
– eosinophils with coarse basophil granules (‘eosinobasophils’, which indicate dyseosinopoiesis)
– increased eosinophil size
– circulating eosinophil myelocytes and/or metamyelocytes
– abnormalities on cytochemical stains
– often a combination of the above e.g. unilobed eosinophils with hypogranulation and many small vacuoles plus eosinophils with trilobed nuclei.
Other morphological features consistent with M-HES include: circulating immature granulocytic cells i.e. neutrophil myelocytes or promyelocytes (but, if blasts >2% are present → CEL), neutrophils with reduced granulation and/or hypersegmented nucleus, increased basophils, monocytosis, anaemia (not iron-deficiency), and thrombocytopenia.
However, in many cases of M-HES there are no abnormal morphological features of eosinophils or increased immature cells in the peripheral blood. Furthermore, the abnormal morphologic features described above are not specific and may be found in reactive eosinophilia e.g. helminthiasis.
Bone marrow examination is important in M-HES, since the bone marrow shows more specific findings than the blood film (i.e. findings consistent with a myeloproliferative disorder): hypercellularity (>70%) due to granulocytic hyperplasia, increased megakaryocytes, clusters of megakaryocytes, marked increase in eosinophils (>20%; their number should be assessed on bone-marrow aspirates aspirates rather than histological sections), bipyramidal Charcot-Leiden crystals (the presence of crystals suggests active eosinophilic inflammation), easily seen blasts (but <5%), increased abnormal spindle-shaped mast cells (ddx: SM), increased reticulin stain (both in amount and quality), and thickening of trabeculae. Increased reticulin is a common, characteristic finding of M-HES.
ΗΕS is morphologically characterised by many dysplastic eosinophils e.g. eosinophils with trilobed nuclei is a typical finding in the blood film
Normal eosinophils, unlike neutrophils, are CD16 negative. An activated eosinophilic phenotype (i.e. high expression of co-stimulatory and cell adhesion markers such as CD23, CD25, CD69, HLA-DR) has been observed with flow cytometry on eosinophils during S. mansoni infection and also in HES and idiopathic hypereosinophilia (iHE). Therefore, flow cytometric measurements of markers cannot differentiate reactive from neoplastic eosinophils.
Tip:
from a practical viewpoint, M-HES is diagnosed when:
- HES + a karyotypic abnormality or molecular abnormality known to cause clonal eosinophilia are present
or - idiopathic HES (eosinophils >1.5×109/l + exclusion of secondary causes + albendazole for 3 days) plus ≥4 of the following 9 clinicopathological features are present:
– dysplastic eosinophils
– vitamin Β12 >1000 pg/ml
– tryptase >12 ng/ml (normal <11.5)
– anaemia (not iron deficiency anaemia) and/or thrombocytopenia
– splenomegaly
– cellularity >80%
– myelofibrosis
– increased mast cells (>10%), of which >25% are spindle shaped
– strong clinical suspicion of a myeloproliferative disorder (FBC, blood film with immature granulocytic cells)
Useful simple tests in patients with hypereosinophilia include serum IgE, vitamin Β12, tryptase, troponin, β2m, CRP, and ESR.
Specialised tests for evaluation of eosinophilia in selected cases e.g. suspected HES:
– Κaryotype
– Flow cytometry/clonogram, PCR for ΤCR gamma and beta gene rearrangements
– FISH or PCR for FIP1L1-PDGFRA, BCR-ABL1, TEL (ETV6)-PDGFRB
– PCR for JAK2V617F mutation
– FISH with PDGFRB, JAK2, FGFR1 break-apart probes
– PCR for KIT D816V mutation, BRAF V600E mutation, DNA sequencing (NGS)
– Extended FISH panel for common clonal abnormalities found in myeloid neoplasms e.g. +8, +9/9q, -5/-5q, -7/-7q, -20q, -13q, -17p, i(17q)
– ANCA auto-antibodies
Figures 4 and 5 illustrate two examples of M-HES. The haematological syndrome of (1) eosinophilia, (2) T-cells with aberrant immunophenotype, (3) detectable TCR clones and (4) clinical manifestations almost exclusively restricted to the skin is characteristic of L-HES. Morphologically, it is characterised by considerable eosinophilia, some degranulated eosinophils, and atypical lymphoid cells with a lower nuclear:cytoplasmic ratio than normal and variable cytoplasmic basophilia resembling immunologically activated lymphocytes. Figure 6 shows an algorithm for the investigation of asymptomatic eosinophilia in travellers or migrants returning from the tropics.13,21
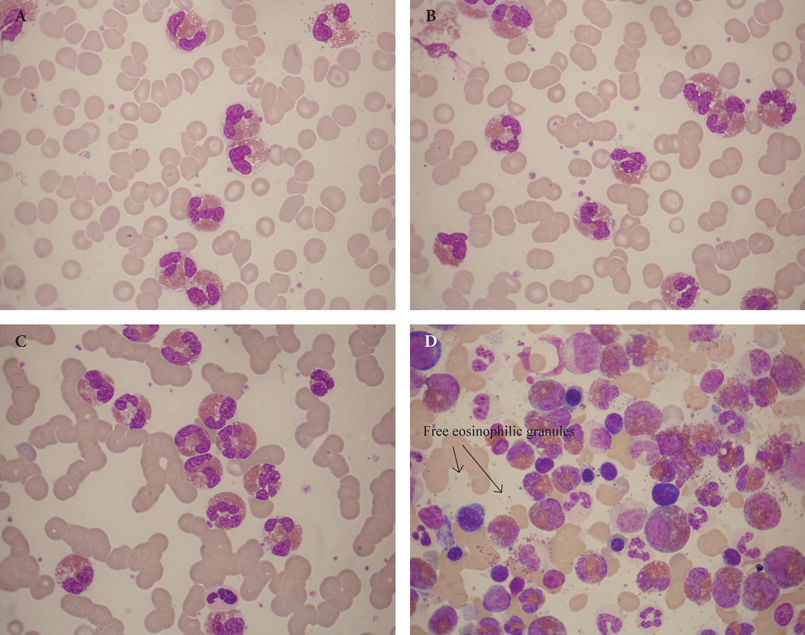
Figure 4. A 65-year-old white English man, with an extensive travel history, presented with anaemia (Hb 8.4 g/dl), arthralgia, WBC 108×109/l (70% eosinophils), PLT 143×109/l. Panels A-C: peripheral blood film showing hypersegmented eosinophils with vacuolation. Panel D: bone marrow aspiration showing increased eosinophil precursors.

Figure 5. A and B. A 16-year-old boy from India with diarrhoea, urticaria, and persistent eosinophilia for one year despite previous albendazole and corticosteroids. Bone marrow showed hypersegmented eosinophils, abnormal granulation (15% of the eosinophils contained purplish granules), and increased numbers of basophils.

Figure 6. Investigation of asymptomatic eosinophilia in returning travellers and migrants.
* A complete serology (ELISA) panel for suspected helminthic infection in a UK reference hospital includes Ascaris Ab, Cysticercosis Ab, Hydatid Ab, Schistosomal Ab, Strongyloides Ab, Toxocara Ab, Trichinella Ab, and Filarial antigen. Schistosomal serology should be repeated after treatment, preferably at 18 months post treatment.
Note:
eosinophilia is generally more common or more pronounced in the initial (acute) phase of the helminthic infection (first 2-6 weeks) and later decreases significantly. However, eosinophilia may be sustained and present in the chronic phase of the infection.
lack of response to seemingly appropriate treatment should prompt assessment for other causes of HES. Following successful treatment of helminthiasis, eosinophilia frequently resolves after a few days, but in some cases it may persist for several weeks or even up to 3 months. Also, a transient exacerbation of eosinophilia may be seen following start of successful treatment in schistosomiasis or filariasis (L. loa and W. bancrofti).
in cases of helminthiasis with eosinophilia >3.0×109/l, the haematological response to antihelminthic treatment is defined as eosinophils <2.0×109/l within 4 weeks.
References
- Peters W, Pasvol G. A colour atlas of tropical medicine and parasitology. 6th ed. London: Mosby Elsevier; c2007.
- Cook GC, Zumla AI. Manson’s tropical diseases. 22nd ed. London: Εlsevier Saunders; c2008.
- Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White N. Manson’s tropical diseases. 23rd ed. London: Εlsevier Saunders; c2014.
- Spira AM. Assessment of travellers who return home ill. Lancet. 2003 Apr;361(9367):1459-69.
- Ryan ET, Wilson ME, Kain KC. Illness after international travel. N Engl J Med. 2002 Aug;347(7):505-16.
- Isba R. Rapid infectious diseases and tropical medicine. Oxford: Blackwell Publishing; c2004.
- Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012 Sep;130(3):607-12.e9.
- Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet. 2019 Jan;393(10167):183-98.
- Butt NM, Lambert J, Ali S, Beer PA, Cross NC. Duncombe A, et al. Guideline for the investigation and management of eosinophilia. Br J Haematol. 2017 Feb;176(4):553-72.
- Hoffbrand AV, Higgs DR, Keeling DM, Mehta AB. Postgraduate haematology. 7th ed. Oxford: Wiley Blackwell; c2016.
- Kaushansky K, Lichtman MA, Prchal JT, Levi MM, Press OW, Burns LJ, et al. William’s hematology. 9th ed. New York: McGraw Hill Education; c2015.
- Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015 Aug;126(9):1069-77.
- Checkley AM, Chiodini PL, Dockrell DH, Bates I, Thwaites GE, Booth HL, et al. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010 Jan;60(1):1-20.
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014 Jun;383(9936):2253-64.
- Jourdan PM, Lamberton PHL, Fenwick A, Addis DG. Soil-transmitted helminth infections. Lancet. 2017 Jan;391(10117):252-65.
- Mazigo HD. Strongyloidiasis and schistosomiasis: lessons from migrants’ data. Lancet Glob Health. 2019 Feb;7(2):e171-2.
- Meunier YA. Tropical Diseases. A practical guide for medical practitioners and students. Oxford: Oxford University Press; c2014.
- Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003 Mar;348(13):1201-14.
- Simon HU, Plotz SG, Dummer R, Blaser K. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med. 1999 Oct;341(15):1112-20.
- Liapis K, Tsagarakis NJ, Panitsas F, Taparkou A, Liapis I, Roubakis C, et al. J Clin Pathol. Epub ahead of print. doi:10.1136/jclinpath-2019-206255.
- Lillie P, McGann H. Empiric albendazole therapy and new onset seizures – a cautionary note. J Infect. 2010 May;60(5):403-4.