Haema 2019; 10(2):66
Konstantinos Liapis
Consultant Haematologist, Georgios Gennimatas Hospital
Full PDF | ![]()
HIV/AIDS is common in the tropics: HIV prevalence is very high in tropical Africa (1-15%), extremely high in South Africa and Botswana (18-20%), and 3.3% in some Caribbean countries (Haiti, Barbados). HIV prevalence is also high in parts of South-East Asia. Thus, HIV has emerged as a major cause of morbidity and mortality in many tropical countries. In many hospitals in East and Southern Africa, over half of all inpatients are infected with HIV, most suffering from complications of HIV.1,2
Haematologists should maintain a high index of suspicion for HIV infection and, from a morphological standpoint, should be aware of the bone marrow changes associated with HIV infection.
Note: HIV-2 infection is endemic in West Africa (HIV-2 generally takes longer to progress to symptomatic HIV/AIDS than HIV-1). HIV is often associated with mild to moderate thrombocytopenia (50-150×109/l), leukopenia, and anaemia. In sub-Saharan Africa, HIV/AIDS is now the commonest cause of anaemia, leukopenia, and thrombocytopenia encountered both in patients and in the community.
The following changes may be seen in the bone marrow of HIV (+) patients with cytopenia:3-6
– hypercellularity
– dysplastic change including dyserythropoiesis, micro-megakaryocytes, and Pelger-Huët anomaly (Figure 1A)
– iron stain consistent with anaemia of chronic disorder (storage iron plentiful with reduced number of sideroblasts)
– reactive plasmacytosis (≥5%), binucleate or even multinucleate plasma cells (Figure 1B)
– increased macrophages, haemophagocytosis, non-specific granulomas, lymphoid aggregates
– reticulin fibrosis
– antiretroviral drug toxicity (e.g. antiretroviral megaloblastic change or hypoplasia)
– hypocellular marrow and gelatinous transformation (in advanced HIV infection)
– infiltration by malignant cells (e.g. acute leukaemia, DLBCL, Burkitt’s lymphoma, Hodgkin’s lymphoma, Castleman’s disease)
– morphological evidence of persistent parvovirus B19 infection i.e. red-cell aplasia/hypoplasia, giant proerythoblasts (‘lantern cells’)
– opportunistic pathogens

Figure 1. Bone marrow changes in ΗΙV infection. A. Dysplastic megakaryocyte (arrow) and dyserythropoiesis (*). B. Increased reactive plasma cells with mature nucleus.
Note: nuclear immaturity and plasmablasts are uncommon in reactive plasmacytosis ≠ plasma cell dyscrasias. Striking bone marrow plasmacytosis in an unwell, febrile HIV (+) patient may indicate Castleman’s disease.
Opportunistic pathogens that involve the bone marrow include:1-7
leishmaniasis
Μycobacterium avium (pseudo-Gaucher cells filled with acid-fast bacilli)
Μycobacterium tuberculosis
Τoxoplasma
Ηistoplasma
Penicillium (in South-East Asia)
rarely other fungi (Cryptococcus, Candida, Pneumocystis, Microsporidia/Encephalitozoon, Blastomyces), and trypanosomes (T. brucei and T. cruzi)
Note: tuberculosis is endemic throughout the tropics.
Mycobacteria cannot be stained with Giemsa (or Gram’s stain) because their thick lipid-rich cell wall prevents stain penetration. With fine focus adjustments, mycobacteria may rarely be visualised in Giemsa stained preparations as unstained, refractile, rod-like structures sometimes called ‘ghost bacilli’ or ‘phantom bacilli’ (arrow), as shown in Figure 2. This case represents an unusual diagnosis, but one that should always be borne in mind when one sees an immunosuppressed patient with pancytopenia. It is worth remembering that miliary tuberculosis may cause myelophthisic changes (leukoerythroblastosis in the blood film and necrotising granulomas with Langhans’ giant cells in the bone marrow) and thrombocytopenia.
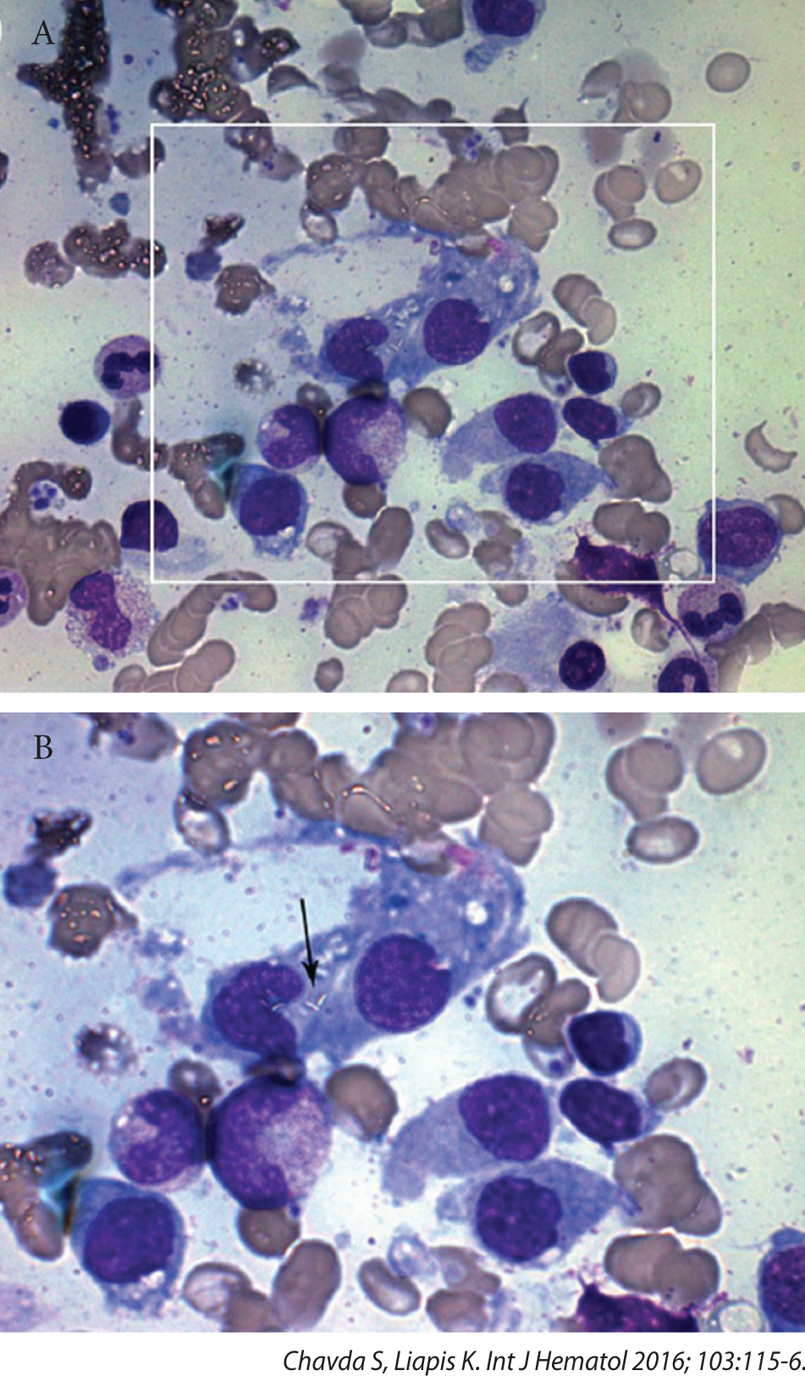 Figure 2. Mycobacterium tuberculosis identified on bone marrow aspiration as ῾ghost bacilli᾿ (arrow).
Figure 2. Mycobacterium tuberculosis identified on bone marrow aspiration as ῾ghost bacilli᾿ (arrow).
Tips:
– maintain a high index of suspicion for HIV and tuberculosis (especially in migrants from high-incidence countries)
– haematological disorders may be the presenting manifestation of HIV infection
– dysplastic changes and cytopenia (‘myelodysplasia’) in young people → consider HIV infection
– severe anaemia in HIV (+) patients should prompt investigation for concomitant tuberculosis
– chronic fever, anorexia, weight loss, splenomegaly and generalised lymphadenopathy in immunocompromised patients should raise the suspicion of tuberculosis
– in immunocompromised patients with the triad: fever, pancytopenia and splenomegaly → request special stains for organisms in bone marrow biopsy sections: Giemsa, Ziehl-Nielsen, periodic acid-Schiff (PAS), Gomori or Grocott, and Gram stain, as seen in Figure 3.

Figure 3. Two HIV (+) patients from India who presented with fever, pancytopenia, splenomegaly, patchy pulmonary infiltrates and generalised lymphadenopathy: Panel A shows disseminated Μ. avium infection (lymph node biopsy; Ziehl-Nielsen stain). Panel B shows disseminated histoplasmosis (bone marrow biopsy; Gomori’s methenamine silver stain).
Gomori’s methenamine silver stain is the preferred stain for demonstrating fungi. Certain fungi demonstrated by Gomori’s stain do not stain well with PAS which should be used as a secondary approach to enhance morphological detail. All Gram (+) bacteria, including the actinomycetes e.g. Actinomyces, Nocardia and mycobacteria stain with Gomori’s as do some encapsulated Gram (-) bacteria e.g. Klebsiella spp. Bacteria that have been treated with antibiotics before tissue sampling may not be well stained with Gram, but they often retain their Gomori positivity. Gomori’s stain also highlights the intracytoplasmic inclusions of CMV-infected cell and the polar bodies of microsporidia.
References
- Cook GC, Zumla AI. Manson’s tropical diseases. 22nd ed. London: Εlsevier Saunders; c2008.
- Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White N. Manson’s tropical diseases. 23rd ed. London: Εlsevier Saunders; c2014.
- Kaushansky K, Lichtman MA, Prchal JT, Levi MM, Press OW, Burns LJ, et al. William’s hematology. 9th ed. New York: McGraw Hill Education; c2015.
- Hoffbrand AV, Higgs DR, Keeling DM, Mehta AB. Postgraduate haematology. 7th ed. Oxford: Wiley Blackwell; c2016.
- Bain BJ, Clark DM, Wilkins BS. Bone marrow pathology. 4th ed. London: Wiley Blackwell; c2011.
- Hoffbrand VA, Pettit JE, Vyas P. Color atlas of clinical hematology. 4th ed. Philadelphia: Mosby Elsevier; c2010.
- Chavda S, Liapis K. Detection of mycobacteria in MGG‑stained bone marrow smears. Int J Hematol. 2016 Feb;103(2):115-6.