Haema 2021; 12(1): 24-29
Konstantinos Liapis
Consultant Haematologist, University Hospital of Alexandroupolis
Full PDF | ![]()
In large areas of the tropics, the problem of feeding the populations and maintaing an adequate status of nutritional health is a serious one, especially that affecting young children. Haematological problems related to the absence of specific nutrients include iron, vitamin B12, and folic acid deficiency. A poor diet usually is not limited to just one nutritional component. Other avitaminoses and protein-calorie malnutrition may also cause haematological abnormalities (iron is immobilised in the reticuloendothelial system in the anaemia of protein-energy malnutrition). The nutritional deficiencies in many cases are complicated further by additional stress imposed by multiple parasitic infections, such as intestinal helminthiases (blood loss), malaria (haemolysis, dyserythropoiesis), tuberculosis (anaemia of chronic disorder, dyserythropoiesis), and HIV (anaemia of chronic disorder, dyserythropoiesis).1-4
Anaemia in the tropics:
- Ascertain if deficiencies occur and define them.
- Ascertain what diseases are accentuating or causing these deficiencies or in any way destroying blood (e.g. hookworm disease, malaria).
- Ascertain what other infections or serious pathological conditions (e.g. tuberculosis, renal disease, hepatic disease, alcohol use, HIV) are present which may inhibit erythropoiesis.
Chronic kidney disease is very common in the tropics; patients often present late, with severe anaemia. In tropical countries, iron deficiency, worm infections and malaria are less common in an urban adult population but may have to be considered in other patients, in particular children, pregnant women or in the rural poor.1-4
The diagnosis of pure iron deficiency anaemia and megaloblastic anaemia is relatively easy from the appearances of peripheral blood and bone marrow.5-8
However, recognition of the co-existence of iron deficiency and megaloblastic anaemia (combined deficiency), a condition which is one of the commonest anaemias of the tropics and of other regions especially in children and women, may be morphologically challenging. Combined deficiency usually results from malnutrition and/or GI tract abnormality. Combined deficiency may also result from chronic alcoholism. In combined deficiency, red cell indices may be normal or abnormal; sometimes MCV and MCH move in opposite directions e.g. high/normal MCV and low MCH (classically macrocytic hypochromic anaemia, or normocytic hypochromic anaemia; less frequently microcytic hypochromic anaemia). The presence of combined hypochromic and megaloblastic anaemia leads to a dimorphic film (‘dimorphic anaemia’).7,9
Dimorphic anaemia in the tropics: an anaemia which is due to two deficiencies, iron deficiency and that of nutritional macrocytic anaemia. It is either iron deficiency anaemia complicated by nutritional macrocytic anaemia or nutritional macrocytic anaemia complicated by iron deficiency. The commonest cause of severe iron deficiency in dimorphic anaemia appears to be a heavy hookworm load and a diet deficient in iron. The commonest cause of the nutritional macrocytic anaemia type of deficiency appears to be due to a diet poor in meat and possibly in green vegetables. The morphology of the blood in dimorphic anaemia shows hypochromic cells (typically concentrated in the central parts of the film) and orthochromic macrocytes (typically collected at the tail). In dimorphic anaemia, erythropoiesis is normocellular. All three types of erythropoiesis can be detected, hypochromic, megaloblastic, and normoblastic, the latter, however, usually predominating. Megaloblastic erythropoiesis is very scanty and may be completely absent. In dimorphic anaemia the peripheral blood and bone marrow show a combination of the following morphological findings:
Definite morphological findings of nutritional deficits:
iron deficiency → 1. empty or significantly reduced iron stores (bone marrow fragments), 2. absence of normal sideroblasts, 3. obvious hypochromic erythrocytes, and 4. cytoplasmic irregularity of late erythroblasts (non-specific).
vitamin B12 or folic acid deficiency → 1. prominent giant metamyelocytes, 2. hypersegmented neutrophils 3. megaloblastic erythropoiesis[1], and 4. macro-ovalocytes.
calorie deficiency → pink staining amorphous material in bone marrow fragments (so-called pink debris) (Figure 1).7,9,10

Figure 1. Pink debris in bone marrow fragments due to malnutrition and significant weight loss.
Iron deficiency anaemia in the tropics:1-4
As normal red cells contain iron as haemoglobin 1 mg per 1 ml, chronic blood loss can lead to negative balance and depletion of iron stores. The aetiology of iron deficiency is as in non-tropical areas (e.g. menorrhagia, aspirin ingestion, peptic ulcers, carcinomas) plus causes common in the tropics including chronic parasitic infection by Ancylostoma duodenale and Necator americanus (hookworm anaemia), Schistosoma species (bilharzial anaemia), and Trichuris trichiura infection, with or without accompanying eosinophilia. Colon cancer and diverticulosis are less common causes of iron deficiency in tropical Africa than in western countries.

Figure 2. Normoblastic erythropoiesis (left column) versus megaloblastic erythropoiesis (right column).
A. duodenale (not to be confused with Ancylostoma caninum and A. braziliense which cause cutaneous larva migrans) hookworms ingest, detach, and digest the duodenal mucosa causing blood loss and/or bleeding. Depletion of iron stores depends on (1) the daily absorption of iron, (2) the size of the body’s iron stores, and (3) the intensity of the hookworm infection i.e. the parasite burden. That is why hookworm anaemia is seen mainly in children and pre-menopausal women (Figure 3). The mechanism of iron deficiency anaemia in T. trichiura is due to inflammation of the colon and blood loss from the inflammed mucosa (T. trichiura infection is prevalent in Central America and in South-East Asia e.g. Malaysia). Iron deficiency anaemia caused by Schistosoma spp. is due to chronic blood loss in stool (chronic Schistosoma colitis, colon polyps, and portal hypertension) and/or in urine (haematuria in S. haematobium).
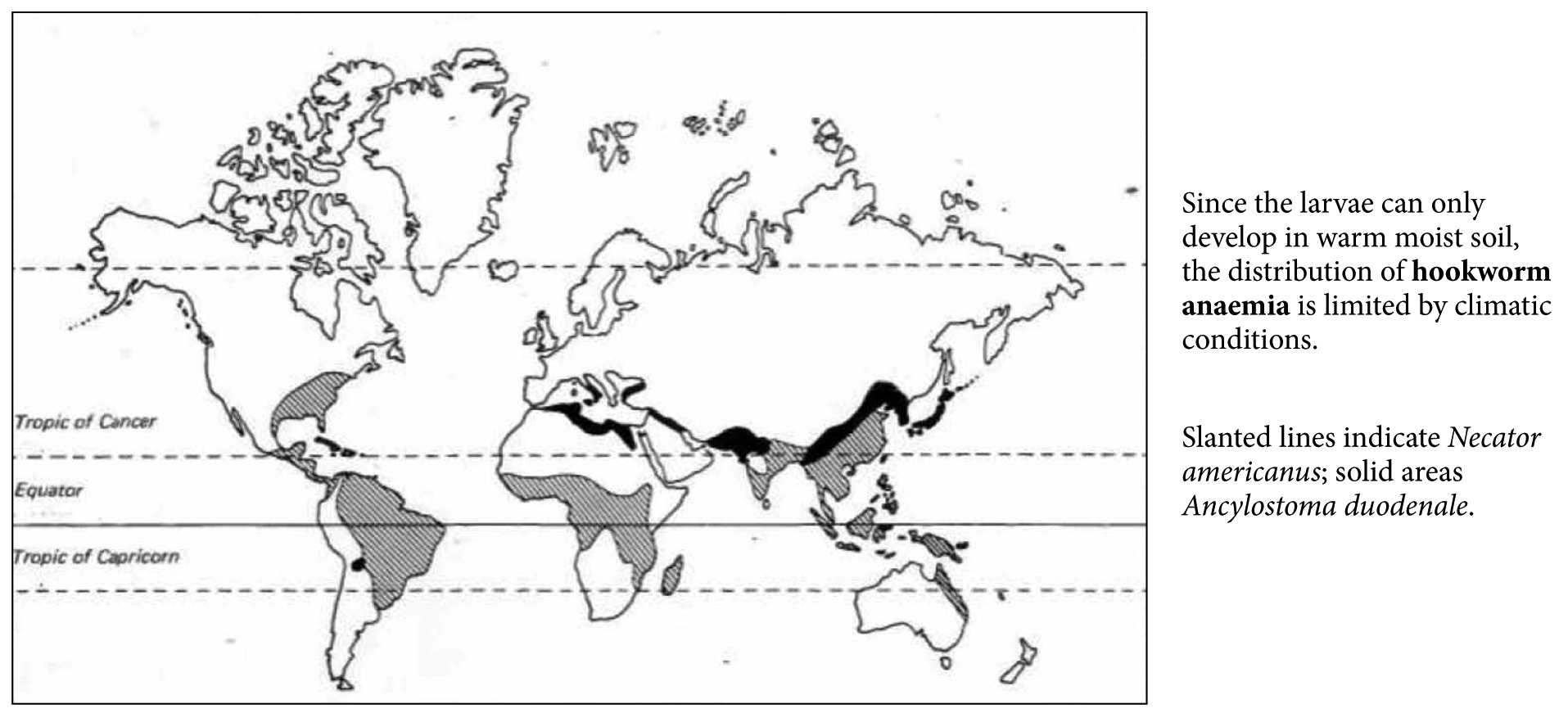
Figure 3. Distribution map of hookworm anaemia.
For investigation of iron deficiency anaemia in migrants from tropical areas especially children and young women, even without gastrointestinal complaints (diarrhoea, abdominal pain, and nausea) or eosinophilia, you should request microscopical examination of stool (or be more pragmatic and consider a trial of albendazole!).
Remember: iron deficiency passes through three stages. In the first stage, iron depletion, iron stores are reduced, but haemopoiesis remains unaffected: serum ferritin is below normal and staining for iron in a bone marrow aspirate shows scanty (1+) or zero iron in the macrophages of a cellular fragment; serum iron is low normal, serum transferrin raised and its percentage saturation reduced within the normal range. In the second stage, iron-deficient erythropoiesis, iron stores have been exhausted: serum ferritin is reduced further, iron is absent from the bone marrow and there are no sideroblasts; serum iron and transferrin saturation are below normal and red cell Zn protoporphyrin is raised; the Hb is likely to be within the normal range or there may be a slight normochromic anaemia. With further depletion, iron is not available for haemoglobin synthesis, and iron deficiency anaemia develops.
In iron deficiency, transferrin saturation is <16% in adults and <14% in children. In contrast, in anaemia of chronic disorders and in protein-energy malnutrition, although saturation of transferrin is decreased, it remains >15%.
In typical α-thalasaemia οr β-thalassaemia trait (common in the tropics), the red cell count is higher (normal-high or >4.9×1012/l), there is less anisocytosis (normal RDW or RDW<18), greater microcytosis, and less hypochromia (normal MCHC) than with iron deficiency. From these characteristics are derived several formulae to differentiate the two conditions; i.e. the Mentzer index:
MCV (fl): Erythrocyte count (×1012/l)
It is typically <13 in β-thalassaemia; in iron deficiency it is >13. However, a Mentzer index between 11-13 may fall into the zone of uncertainty. Another useful formula that could distinguish thalassaemia trait from other microcytic anaemias is England and Fraser’s discriminant function (D.F.).11-13
The presence of basophilic stippling and a large number of target cells are typical characteristics of β-thalassaemia ≠ iron deficiency anaemia. β-thalassaemia should also be considered if there is no response or incomplete response to iron supplementation. Typical iron studies in iron deficiency anaemia include: MCV<80 fl, serum ferritin <20 ng/ml, low iron (<65 μg/dl), and high TIBC (>400 μg/dl).
Megaloblastic anaemia in the tropics:1-4,14,15
The aetiology of megaloblastic anaemia is as in non-tropical areas plus a common cause in the tropics is malabsorption of folic acid resultsing from tropical sprue and from acute or chronic intestinal infections (especially giardiasis).
Note: folate is found in green vegetables and fruits. Folic acid deficiency can result from decreased intake, impaired absorption, and increased utilisation of folate, although the commonest cause in the tropics is dietary insufficiency.
Tropical sprue (or post-infective tropical malabsorption) is an important cause of megaloblastic anaemia in the tropics.3,16,17 It consists of chronic diarrhoea (>3 weeks), steatorrhoea, and folic acid deficiency (macrocytosis with or without anaemia) in people who have returned from tropical areas particularly India (Figure 4).
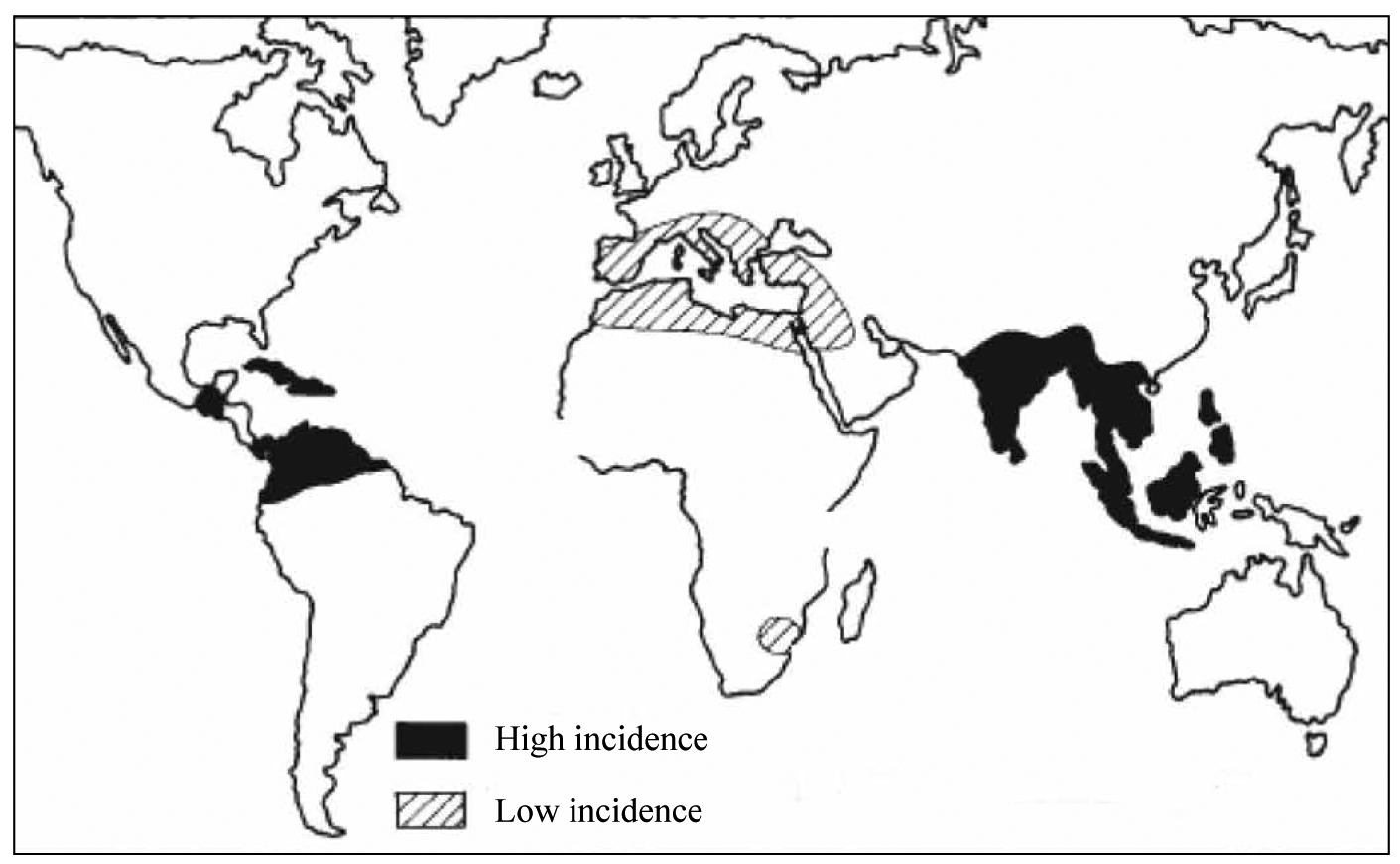
Figure 4. Distribution map of tropical sprue.
Tropical sprue is rare in tropical Africa, but has been reported from North Africa (Egypt). Fever is not part of the picture. It is probably due to colonisation of jejenum by aerobic enterobacteria E. coli, Klebsiella, and Enterobacter. Folic acid deficiency plays a central role in the pathophysiology. The bone marrow is normocellular with megaloblastic change, which is more obvious in the granulocytic series (giant metamyelocytes). Iron, serum ferritin, vitamin B12, albumin, electrolytes, and fluid balance are usually unaffected.
The diagnosis depends upon finding low serum folic acid and/or red cell folate (although red cell folate falls below normal more slowly than serum folic acid [because t1/2 red cells: 120 days], it is a better marker of tissue folic acid levels. Red cell folate is falsely low in vitamin B12 deficiency and falsely increased above normal during malaria, possibly due to synthesis of folic acid by the parasites themselves).1-4

Figure 5. Panels A-E. Megaloblastic change in tropical sprue. Iron stain was normal (Panel F).
The differential diagnosis of tropical sprue includes giardiasis (giardiasis is the parasitic infection most commonly associated with malabsorption: acute diarrhoea due to Giardia causes a malabsorption of folic acid, whereas about half of the patients with chronic giardiasis have impaired absorption of vitamin B12 due to utilisation of the vitamin by the parasite and bacterial overgrowth of the bowel), Strongyloides (the second most common parasite associated with malabsorption), Capillaria philippinensis, immunoproliferative small intestinal disease (IPSID), pellagra, and non-tropical conditions (e.g. Crohn’s disease, Whipple’s disease, coeliac disease, common variable immunodeficiency). In immunocompromised patients (e.g. HIV+, bone-marrow or solid organ transplantation) à also consider CMV, Cryptosporidium, Microsporidium, Cyclospora, Isospora, and lymphoma.2-4,18-21
Remember: wasting and anaemia in an HIV (+) patient suggest advanced immunosuppression and/or possible concomitant tuberculosis.
The following case is an example of tropical sprue. A 33-year-old man presented with macrocytosis (ΜCV 103 fl), Hb 13 g/dl, diarrhoea, and 12 kg weight loss after returning from a journey to India 2 months earlier. He was found to have WBC 6.0×109/l, PLT 290×109/l, hypersegmented neutrophils, normal eosinophil count, malaria parasites (-), albumin 3.6 g/dl, electrolytes normal, HIV (-), stool microscopy (-), stool culture (-), ferritin 200 ng/ml, vitamin B12 322 pg/ml, and serum folic acid 2.2 ng/ml (normal, 1.5-5.5). Bone marrow aspiration showed megaloblastic change (Figure 5). Red cell folate was 114 ng/l (normal >150 ng/l) and fecal fat was positive consistent with steatorrhoea.
References
- Peters W, Gilles HM. A colour atlas of tropical medicine and parasitology. 2nd ed. London: Wolfe; c1981.
- Peters W, Pasvol G. A colour atlas of tropical medicine and parasitology. 6th ed. London: Mosby Elsevier; c2007.
- Cook GC, Zumla AI. Manson’s tropical disease. 22nd ed. London: Εlsevier Saunders; c2008.
- Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White N. Manson’s tropical diseases. 23rd ed. London: Εlsevier Saunders; c2014.
- World Health Organization. Βench aids for the morphological diagnosis of anaemia. Geneva: WΗO; c2001.
- Bain BJ. Blood cells – a practical guide. 3rd ed. London: Blackwell Publishing; c2002.
- Kapff C, Jandl J. Blood atlas and sourcebook of hematology. 2nd ed. Boston: Little Brown and Company; c1991.
- O’Connor BH. A color atlas and instruction manual to peripheral blood cell morphology. 1st ed. Philadelphia: Lippincott Williams & Wilkins; c1984.
- Trowell HC. The morphology of the blood in dimorphic anaemia. Trans R Soc Trop Med Hyg. 1942 Nov;36(3):151-76.
- Savage D, Lindenbaum J. Anemia in alcoholics. Medicine (Baltimore). 1986 Sep;65(5):322-38.
- England JM, Fraser PM. Differentiation of iron deficiency from thalassaemia trait by routine blood-count. Lancet. 1973 Mar;1(7801):449-52.
- Mentzer Jr WC. Differentiation of iron deficiency from thalassaemia trait. Lancet.1973 Apr;1(7808):882.
- AlFadhli SM, Al-Awadhi AM, AlKhaldib D. Validity assessment of nine discriminant functions used for the differentiation between iron deficiency anemia and thalassemia minor. J Trop Pediatr. 2007 Apr;53(2):93-7.
- Bawaskar HS, Bawaskar PH, Bawaskar PH, Parekh PB. Tropical megaloblastic anaemia. Lancet. 2019 Jun;393(10187):2261.
- Bedu-Addo G, Ampem Amoako Y, Bates I. The role of bone marrow aspirate and trephine samples in haematological diagnoses in patients referred to a teaching hospital in Ghana. Ghana Med J. 2013 Jun;47(2):74-8.
- Cook GC. Aetiology and pathogenesis of postinfective tropical malabsorption (tropical sprue). Lancet. 1984 Mar;1(8379):721-3.
- Glynn J. Tropical sprue: its aetiology and pathogenesis. J R Soc Med. 1988 Oct;79:599–606.
- Jelinek T, Peyerl G, Löscher T, Nothdurft HD. Giardiasis in travellers: evaluation of an antigen-capture ELISA for the detection of Giardia lamblia-antigen in stool. Z Gastroenterol. 1996 Apr;34(4):237-40.
- Mathan VI. Tropical sprue in southern India. Version 2. Trans R Soc Trop Med Hyg. 1988;82(1):10-4.
- Ghoshal UC, Chetri K, Banerjee PK, Choudhuri G, Pal BB, Dabadghao S, et al. Is immunoproliferative small intestinal disease uncommon in India? Trop Gastroenterol. 2001 Jan-Mar;229(1):14-7.
- Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroent. 2007 Apr;13(15):2153-9.
[1] Megaloblastic anaemia can be masked by co-existing iron deficiency anaemia or thalassaemia. The characteristic features of megaloblastic erythropoiesis are shown in Figure 2.8